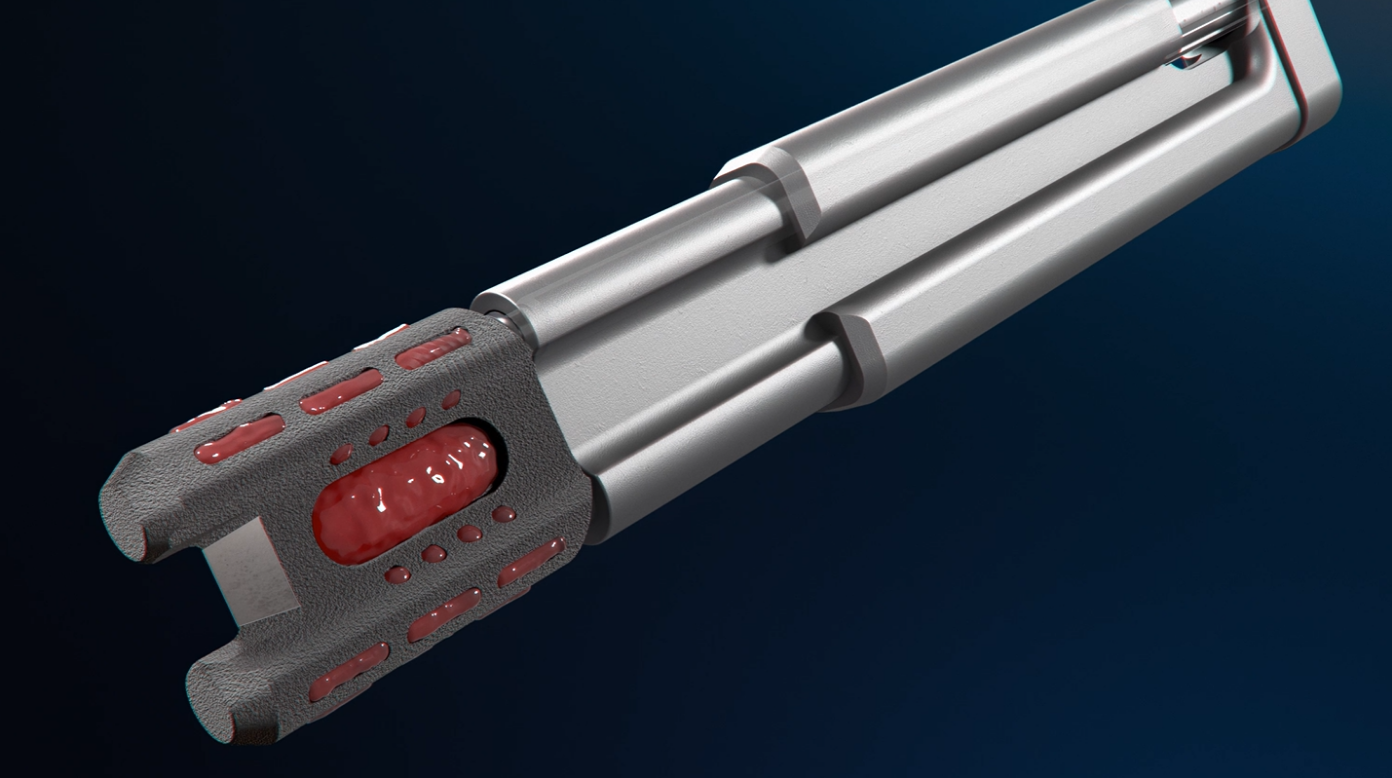
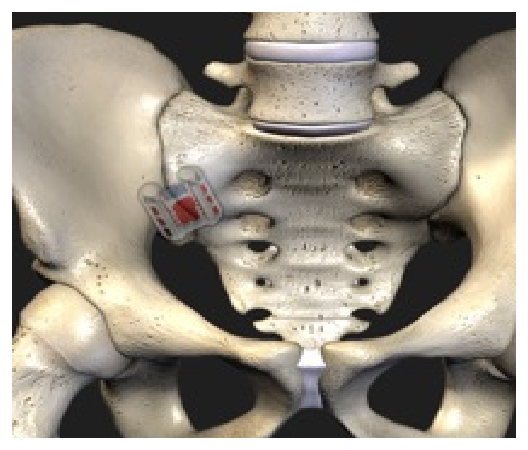
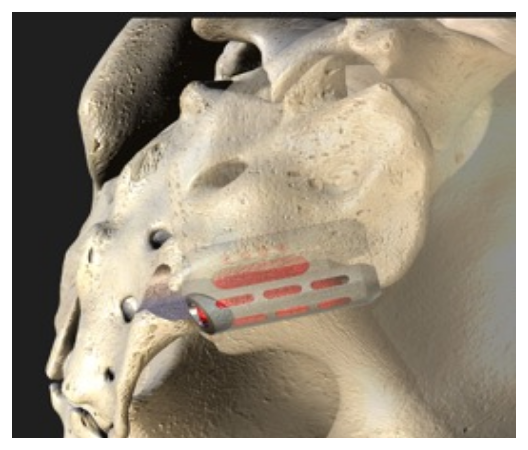
The Catamaran® SI Joint Fusion System offers a novel, less invasive Inferior-Posterior approach to the SI joint using a single, robust titanium implant.
The System features the Catamaran® Fixation Device, which passes through both the axial and sagittal planes of the ilium and sacrum, transfixing the SI joint along its longitudinal axis.
A Better Option for SI Joint Fusion
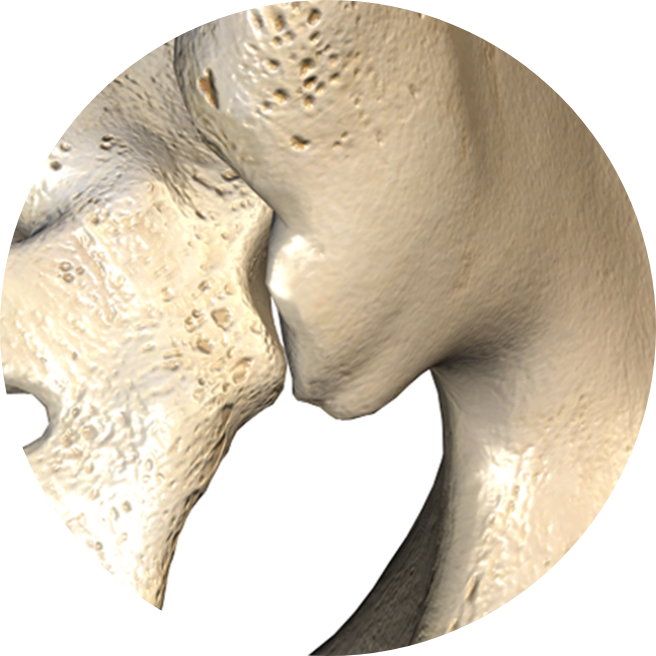
Favorable Entry
Direct access to the SI joint provides optimal entry point to facilitate arthrodesis
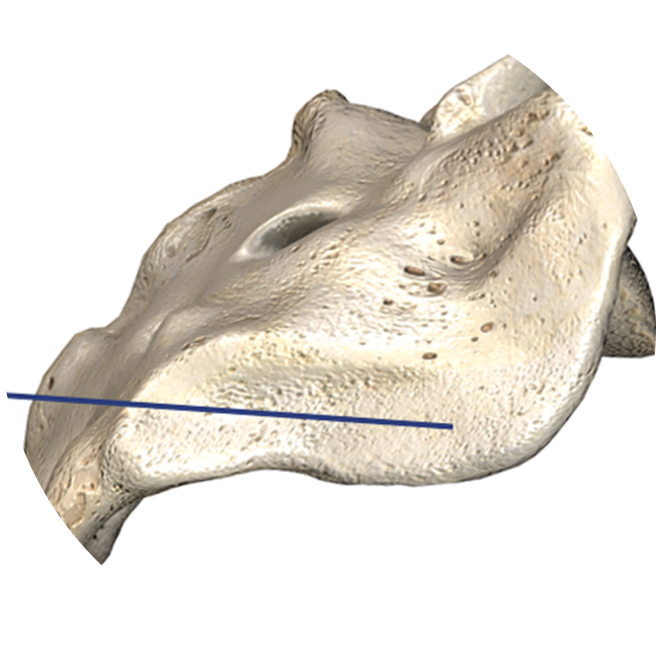
Inferior-Posterior Approach
Trajectory is angled away from critical neural and vascular structures
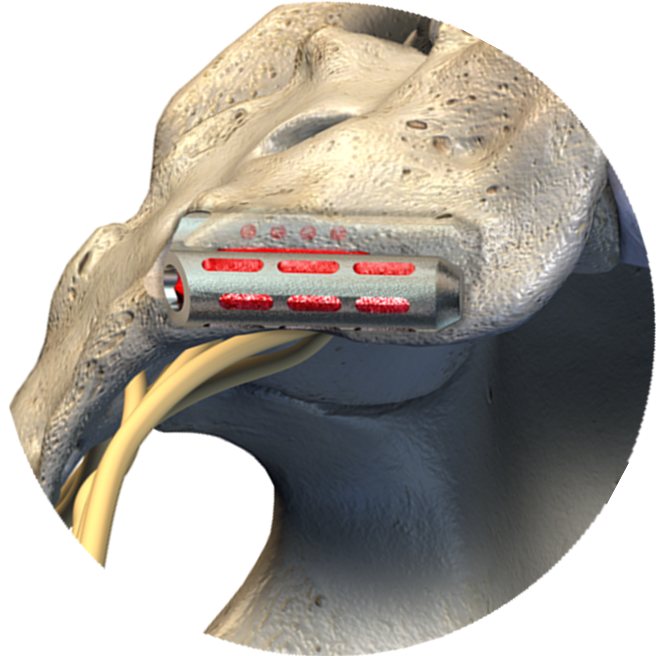
Optimized Placement
Designed for maximum fixation in the dense cortical bone of the SI joint, inferior to the dorsal recess

Single, Robust
Titanium Implant
A single implant that transfixes the SI joint
The Catamaran SI Joint Fusion System is intended for sacroiliac joint fusion for conditions including sacroiliac joint disruptions and degenerative sacroiliitis

Utilizing a 2D fluoroimaging sequence, the Catamaran Fixation Device is implanted with one pontoon fixated into the ilium and the other into the sacrum, transfixing the SI joint along its longitudinal axis.
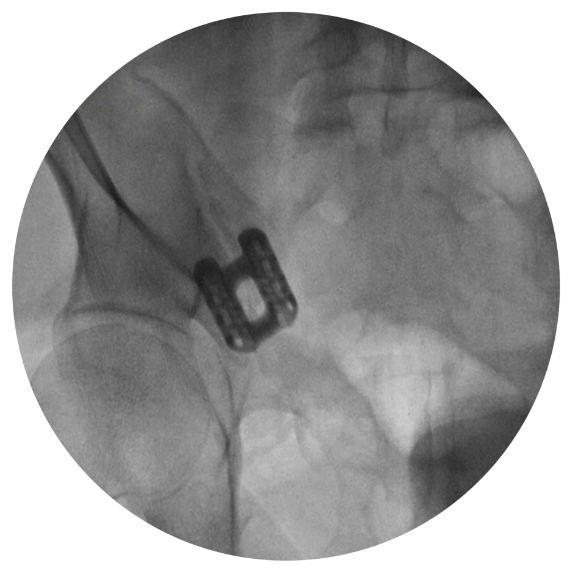
MODIFIED OUTLET VIEW
Implant Trajectory
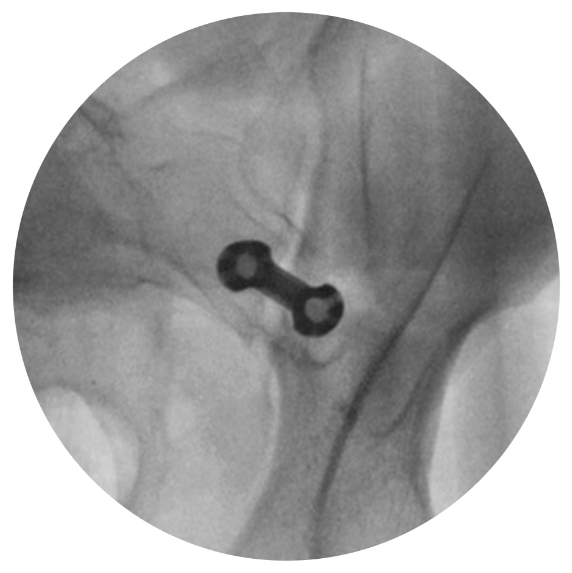
MODIFIED INLET VIEW
Implant Entry
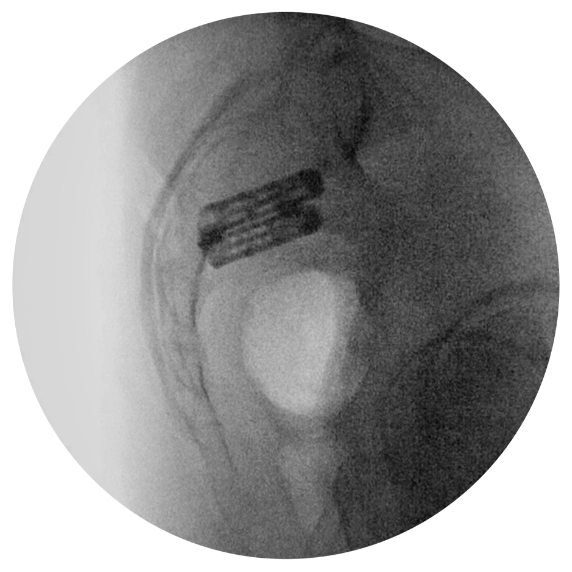
LATERAL
VIEW
Implant Depth
Catamaran SI Joint Fixation
Device:
Design & Specifications
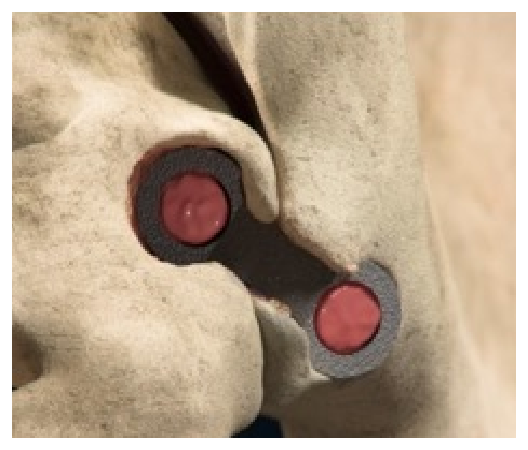
Joined by a patented transfixing osteotome bridge, the implant consists of two hollow fenestrated pontoons with an open framework designed for packing with autologous bone to facilitate bony in-growth through the SI joint.
The transfixing osteotome is designed to disrupt the articular portion of the joint to help facilitate a fusion response.

Catamaran Biomechanical Characterization and Testing
In mechanical testing, the Catamaran Fixation Device performed favorably as a reliable sacroiliac joint fixation/stabilization system that greatly exceeded the biomechanical thresholds of the sacroiliac joint.
White Paper PDF: Performance Integrity of the Catamaran SI Joint Fixation Device in a Novel, Inferior-Posterior Approach for Sacroiliac Joint Fusion

If you would prefer a printed copy of any of these documents sent to you, please contact Tenon Customer Service at 1-844-211-2540.
A Single Center Experience with the
Catamaran SI Joint Fusion System
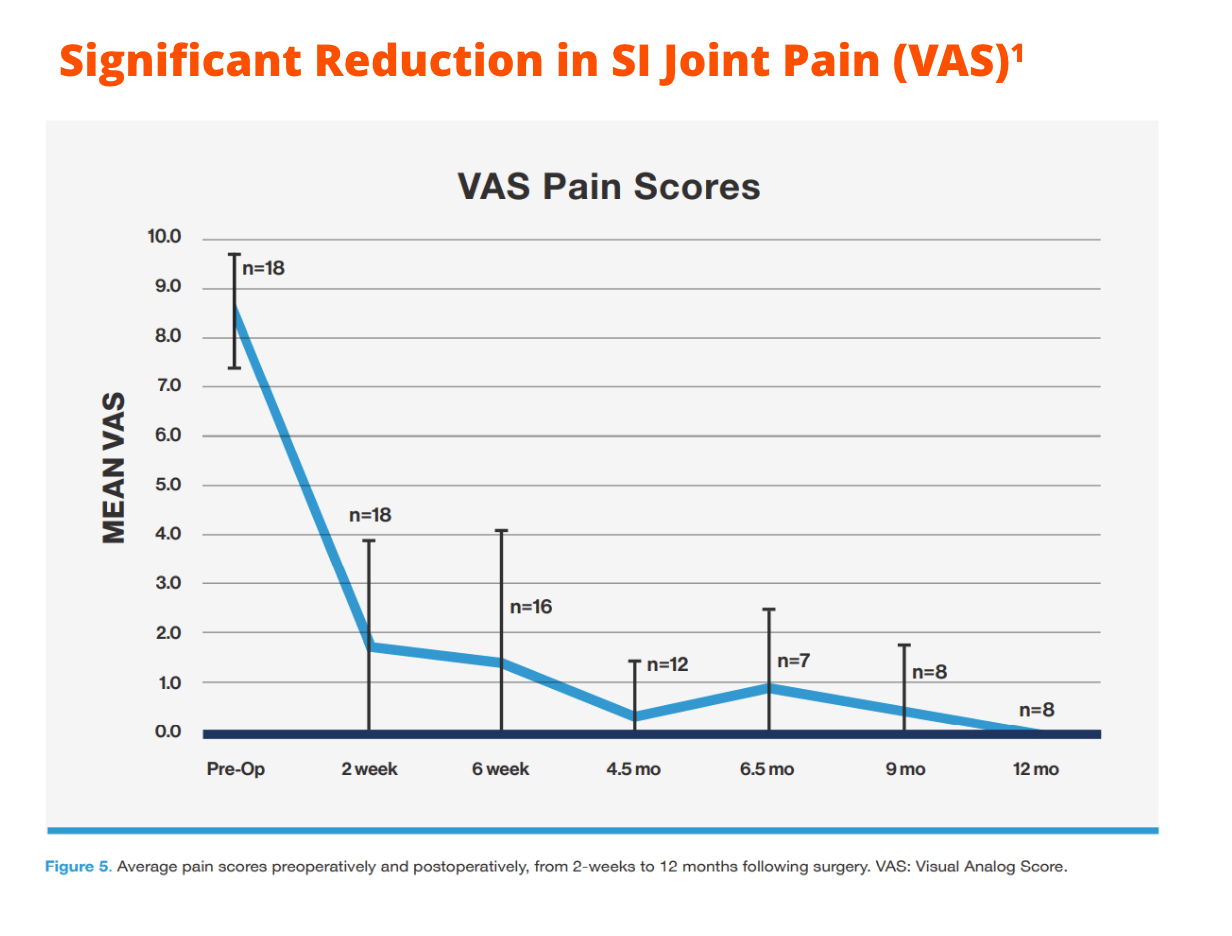
Early clinical experience has shown that the Catamaran® SI Joint Fixation Device via an inferior-posterior approach has the potential to deliver significant & sustained reduction in SI joint pain, as well as:
White Paper PDF: SACROILIAC JOINT FUSION: Improving Surgical Outcomes Through an Inferior-Posterior Approach with the Tenon Medical Catamaran SI Joint Fusion System
Stay Updated
To learn more about the Catamaran SI Joint Fusion System and discuss training opportunities, please complete the form below and a representative will contact you.